Biopharmcatalyst Fda Calendar
Biopharmcatalyst Fda Calendar - Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline. Web our fda calendar track catalysts in every stage of drug development, from phase 1 to approval. Web biopharmcatalyst provides a pharmaceutical data bank that keeps track of biotech stocks, fda approvals, advisory committee activity, pdufa and phase 2 & 3 trial data. Web comprehensive suite of tools for trading and investing in biotech stocks. Fda calendar, pdufa date calendar, biotech company screener and database and much more With over 1200+ catalysts listed our filtering feature, allows clients to pick. Web pdufa dates and fda panel review dates are very important catalysts because they are ‘make or break events’ for biotech stocks. The goal date set by the fda for. Web biopharmcatalyst provides a pharmaceutical data bank that keeps track of biotech stocks, fda approvals, advisory committee activity, pdufa and phase 2 & 3 trial data. The goal date set by the fda for. With over 1200+ catalysts listed our filtering feature, allows clients to pick. Web comprehensive suite of tools for trading and investing in biotech stocks. Web our. Fda calendar, pdufa date calendar, biotech company screener and database and much more Web comprehensive suite of tools for trading and investing in biotech stocks. With over 1200+ catalysts listed our filtering feature, allows clients to pick. The goal date set by the fda for. Web our fda calendar track catalysts in every stage of drug development, from phase 1. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline. Web our fda calendar track catalysts in every stage of drug development, from phase 1 to approval. Web comprehensive suite of tools for trading and investing in biotech stocks. With over 1200+ catalysts listed our filtering feature, allows. Web pdufa dates and fda panel review dates are very important catalysts because they are ‘make or break events’ for biotech stocks. Web our fda calendar track catalysts in every stage of drug development, from phase 1 to approval. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single. Web biopharmcatalyst provides a pharmaceutical data bank that keeps track of biotech stocks, fda approvals, advisory committee activity, pdufa and phase 2 & 3 trial data. The goal date set by the fda for. Fda calendar, pdufa date calendar, biotech company screener and database and much more Web pdufa dates and fda panel review dates are very important catalysts because. The goal date set by the fda for. Web our fda calendar track catalysts in every stage of drug development, from phase 1 to approval. With over 1200+ catalysts listed our filtering feature, allows clients to pick. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline. Web. With over 1200+ catalysts listed our filtering feature, allows clients to pick. Web biopharmcatalyst provides a pharmaceutical data bank that keeps track of biotech stocks, fda approvals, advisory committee activity, pdufa and phase 2 & 3 trial data. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline.. With over 1200+ catalysts listed our filtering feature, allows clients to pick. Web comprehensive suite of tools for trading and investing in biotech stocks. Fda calendar, pdufa date calendar, biotech company screener and database and much more The goal date set by the fda for. Web pdufa dates and fda panel review dates are very important catalysts because they are. Web comprehensive suite of tools for trading and investing in biotech stocks. Web pdufa dates and fda panel review dates are very important catalysts because they are ‘make or break events’ for biotech stocks. Web biopharmcatalyst provides a pharmaceutical data bank that keeps track of biotech stocks, fda approvals, advisory committee activity, pdufa and phase 2 & 3 trial data.. With over 1200+ catalysts listed our filtering feature, allows clients to pick. Web our fda calendar track catalysts in every stage of drug development, from phase 1 to approval. Web biopharmcatalyst provides a pharmaceutical data bank that keeps track of biotech stocks, fda approvals, advisory committee activity, pdufa and phase 2 & 3 trial data. Fda calendar, pdufa date calendar,. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline. Web biopharmcatalyst provides a pharmaceutical data bank that keeps track of biotech stocks, fda approvals, advisory committee activity, pdufa and phase 2 & 3 trial data. Fda calendar, pdufa date calendar, biotech company screener and database and much more Web comprehensive suite of tools for trading and investing in biotech stocks. With over 1200+ catalysts listed our filtering feature, allows clients to pick. Web our fda calendar track catalysts in every stage of drug development, from phase 1 to approval. Web pdufa dates and fda panel review dates are very important catalysts because they are ‘make or break events’ for biotech stocks. The goal date set by the fda for.FDA 캘린더 일정을 달력으로 보여주는 사이트 바이오 주식
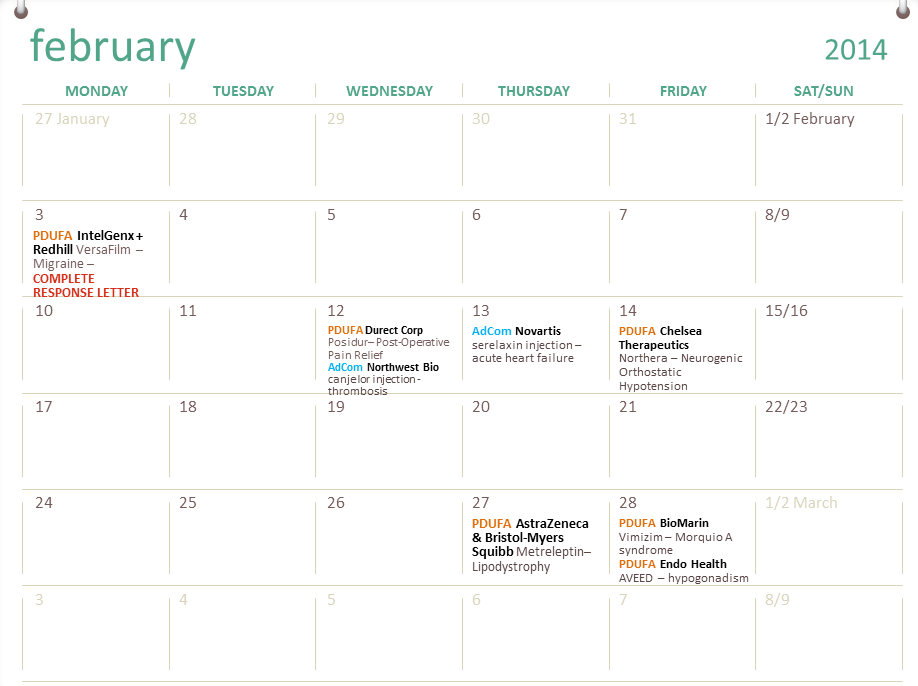
FDA Calendar FDA Tracker
Cytodyn Inc. (CYDY) The Company filed its BLA for Leronlimab as
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
FDA Calendar FDA Tracker
FDA Academy Course Calendar 2013 Pharmacovigilance Food And Drug
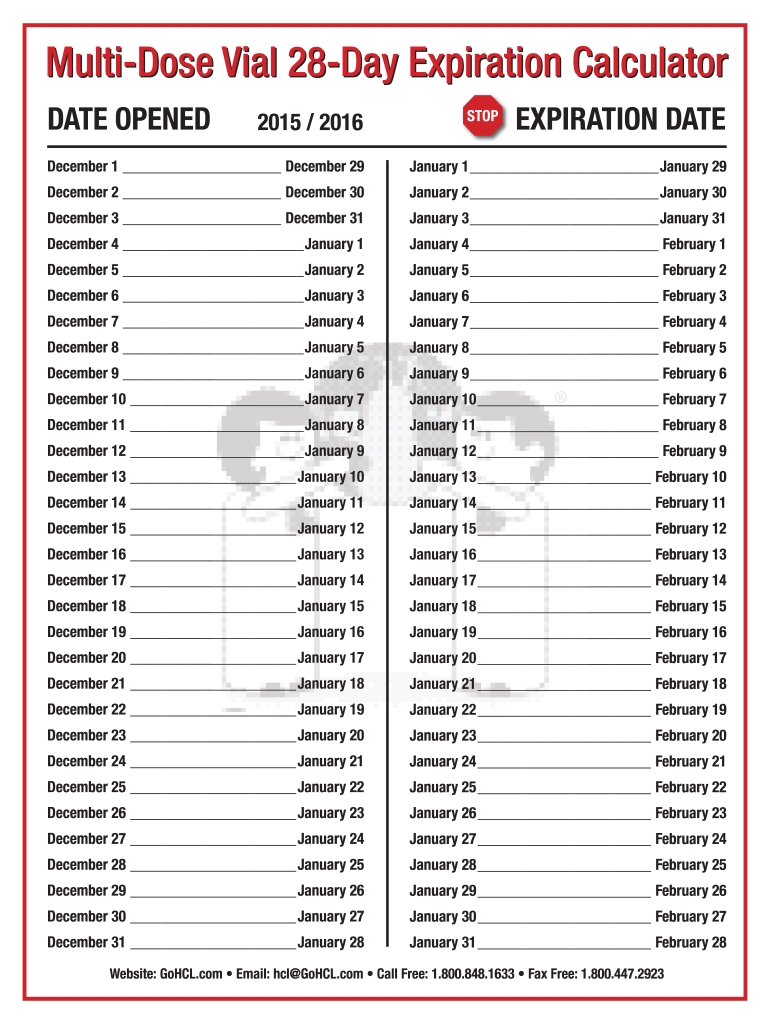
How to 28 Day Calendar For Multi Dose Medications Get Your Calendar
FDA Calendar FDA Tracker
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
FDA Calendar FDA Tracker
Related Post: