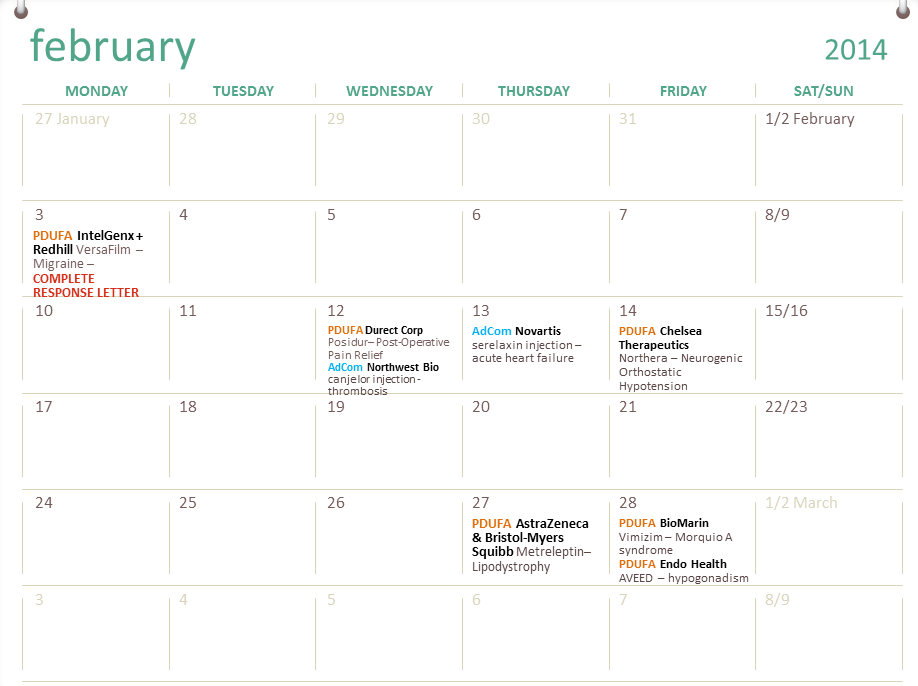
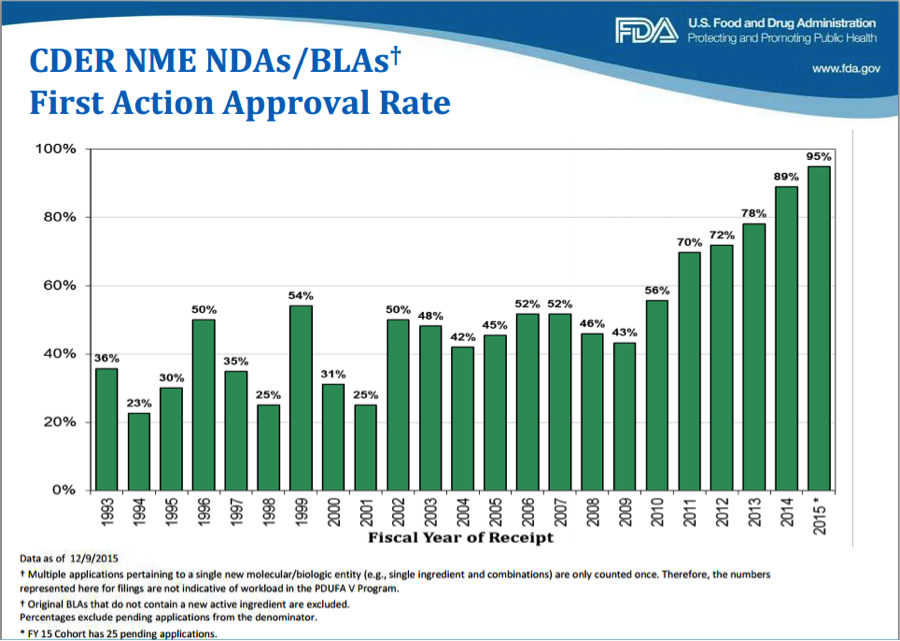
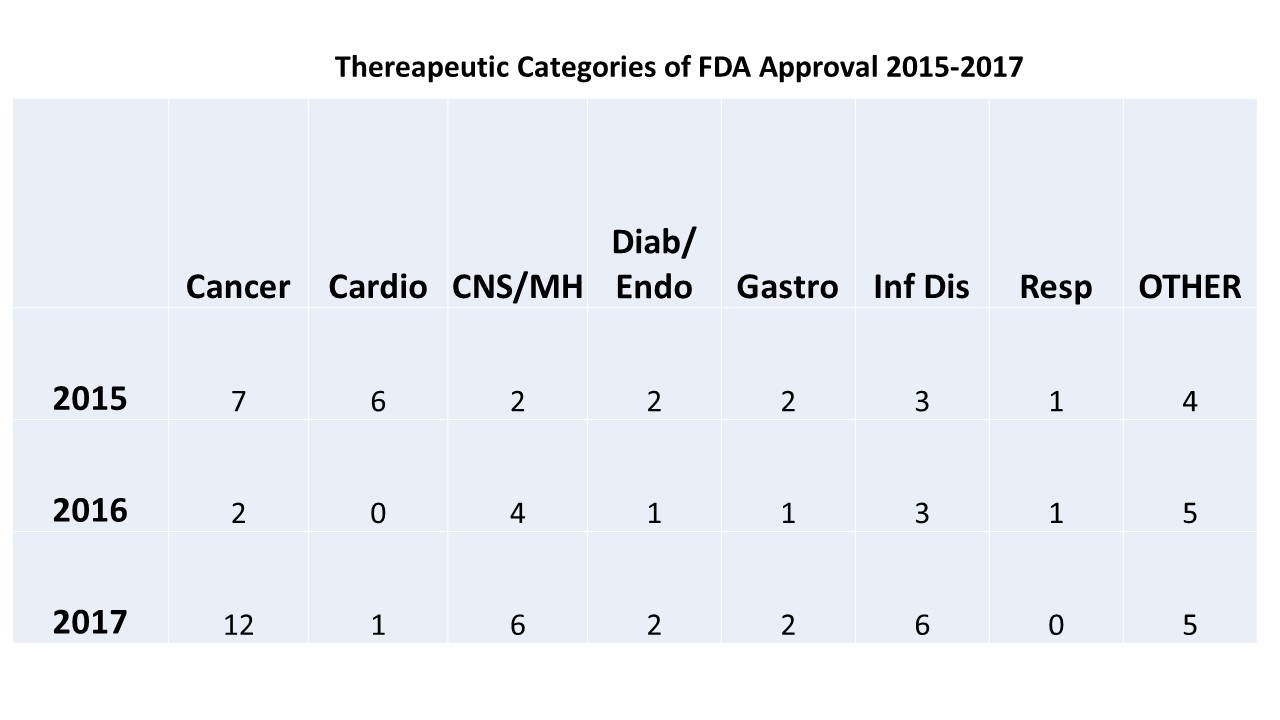
Fda Approval Calender
Fda Approval Calender - It lists significant meetings held by designated. Phase 3 topline data are anticipated in the week of july 10, 2023 with nda filing due. The fda pdufa calendar is a chronological calendar of pdufa target dates as well as advisory meetings. Web the fda approved 37 novel drugs in 2022, the fewest to pass regulatory scrutiny since 2016. Web us fda approval and panel tracker: Updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the. Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews. Web approval date nda 211929 ortikos budesonide sun pharma global fze s 6/13/2019 1/30/2019, 4/18/2019 nda. Web significant meetings held by fda officials with persons outside of the executive branch of the federal. Last month the fda granted. Phase 3 topline data are anticipated in the week of july 10, 2023 with nda filing due. Web this public calendar is issued by the food and drug administration. Web fda approval date means the date of receipt of fda approval allowing for patheon ’s manufacturing, testing, and packaging for. Last month the fda granted. Web the fda approved 37. Web 2023 device approvals. The fda pdufa calendar is a chronological calendar of pdufa target dates as well as advisory meetings. Cder drug and biologic approvals for calendar year 2022. Web g et ready for a deluge of important approval decisions. Updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the. Web fda approval date means the date of receipt of fda approval allowing for patheon ’s manufacturing, testing, and packaging for. Our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Cder drug and biologic approvals for. It lists significant meetings held by designated. It lists significant meetings held by designated. The fda pdufa calendar is a chronological calendar of pdufa target dates as well as advisory meetings. Web the pdufa/fda approval calendar and finding potential fda approval catalyst dates biopharmcatalyst provides a. Cder drug and biologic approvals for. Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews. Web 52 rows. Web advisory committee calendar print this page contains notices of advisory committee meetings. Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews. Web us fda approval and panel tracker: Web phase 3 last patient dosed on may 22, 2023. Web get informed of the current and upcoming fda approved drugs,. Web get informed of the current and upcoming fda approved drugs, meetings, and more with this comprehensive guide to the fda. Web 2023 device approvals. Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. The products listed in this section include some of the newest medical technology from the.. Our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. This year, before april showers get a chance to bring. Web fda approval date means the date of receipt of fda approval allowing for patheon ’s manufacturing, testing, and packaging for. Web this public calendar is issued by the food and drug administration. Web phase. Web the fda approved 37 novel drugs in 2022, the fewest to pass regulatory scrutiny since 2016. Our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Last month the fda granted. Web the pdufa/fda approval calendar and finding potential fda approval catalyst dates biopharmcatalyst provides a. The fda pdufa calendar is a chronological calendar. Web what is an fda calendar? Cder drug and biologic approvals for calendar year 2022. Updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the. It lists significant meetings held by designated. Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews. Cder drug and biologic approvals for calendar year 2022. Phase 3 topline data are anticipated in the week of july 10, 2023 with nda filing due. Web significant meetings held by fda officials with persons outside of the executive branch of the federal. This year, before april showers get a chance to bring. It lists significant meetings held by designated. Web us fda approval and panel tracker: Cder drug and biologic approvals for. This year, before april showers get a chance to bring. Web the fda approved 37 novel drugs in 2022, the fewest to pass regulatory scrutiny since 2016. Web this public calendar is issued by the food and drug administration. Web get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews. Web advisory committee calendar print this page contains notices of advisory committee meetings. Web what is an fda calendar? Web g et ready for a deluge of important approval decisions. Phase 3 topline data are anticipated in the week of july 10, 2023 with nda filing due. Our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web 2023 device approvals. Updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the. The products listed in this section include some of the newest medical technology from the. Web phase 3 last patient dosed on may 22, 2023. Web this public calendar is issued by the food and drug administration. It lists significant meetings held by designated. It lists significant meetings held by designated. Cder drug and biologic approvals for calendar year 2022.Fda Approval Calendar Qualads
FDA Calendar FDA Tracker
FADIC Calender
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
The Most Important New Drug Of 2014
Fda Generic Approvals 2015 takvim kalender HD
Is FDA Set for Another Banner Year of Approvals? Eye on FDA
FDA Calendar FDA Tracker
Pin on calendar Printable Online 2019
Related Post: