Fda Approvals Calendar
Fda Approvals Calendar - Web the cder fast track (ft) approvals reports contain a list of approvals for fast track designated drugs. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Business appears to be back to normal at the fda. Web fda calendar start free trial our fda calendar is designed to provide you with future catalysts across biotech & pharma. Web get informed of the current and upcoming fda approved drugs, meetings, and more with this comprehensive guide to the fda. Patrizia cavazzoni, m.d., director, center for drug evaluation and research. Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. This public calendar is issued by the food and drug administration. May 2023 joanne fagg madeleine armstrong last month the fda granted. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Cder drug and biologic approvals for calendar year. Web fda decisions (approvals/complete response letter/delay) according to special statuses for treatment options, including orphan. Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Et ready for a deluge of important approval. Web 2023 device approvals. This public calendar is issued by the food and drug administration. Web this public calendar is issued by the food and drug administration. Web updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the fda is expected to. Web 52 rows below is a listing of new molecular entities and new therapeutic biological. Phase3.bio places the fda review team’s deliberative process for drug evaluation into perspective so. Web fda calendar start free trial our fda calendar is designed to provide you with future catalysts across biotech & pharma. Web get informed of the current and upcoming fda approved drugs, meetings, and more with this comprehensive guide to the fda. Cder drug and biologic. May 2023 joanne fagg madeleine armstrong last month the fda granted. Patrizia cavazzoni, m.d., director, center for drug evaluation and research. Business appears to be back to normal at the fda. Web us fda approval and panel tracker: David kaslow said in june at the. It lists significant meetings held by designated. This public calendar is issued by the food and drug administration. The products listed in this section include some of the newest medical technology from the. In the second quarter of 2023, the agency. Web cder drug and biologic approvals for calendar year 2021; Web updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the fda is expected to. Jan 9, 2022 6:24am est. Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web cder drug and biologic approvals for calendar year 2021; Web this public. Web 3 big fda approvals to watch for in q1 2022. Web at the time of approval, fda and sponsors agree on target timelines for completion of study milestones, such as. This public calendar is issued by the food and drug administration. Web cder drug and biologic approvals for calendar year 2021; David kaslow said in june at the. Web 2023 device approvals. Phase3.bio places the fda review team’s deliberative process for drug evaluation into perspective so. Web us fda approval and panel tracker: Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Web cy 2020 fast track calendar year approvals*. This public calendar is issued by the food and drug administration. Web the cder fast track (ft) approvals reports contain a list of approvals for fast track designated drugs. Web the pdufa/fda approval calendar and finding potential fda approval catalyst dates biopharmcatalyst provides a. Web cy 2019 cder drug and biologic calendar year approvals as of december 31, 2019 this. Patrizia cavazzoni, m.d., director, center for drug evaluation and research. Web february 22, 2022. Web fda calendar start free trial our fda calendar is designed to provide you with future catalysts across biotech & pharma. Web cder drug and biologic approvals for calendar year 2021; It lists significant meetings held by designated. Patrizia cavazzoni, m.d., director, center for drug evaluation and research. The products listed in this section include some of the newest medical technology from the. Web cder drug and biologic approvals for calendar year 2021; It lists significant meetings held by designated. Web updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the fda is expected to. Web the pdufa/fda approval calendar and finding potential fda approval catalyst dates biopharmcatalyst provides a. Web cy 2020 fast track calendar year approvals*. Web us fda approval and panel tracker: Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web 52 rows below is a listing of new molecular entities and new therapeutic biological products that cder approved in. This public calendar is issued by the food and drug administration. Web this public calendar is issued by the food and drug administration. Web fda decisions (approvals/complete response letter/delay) according to special statuses for treatment options, including orphan. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Business appears to be back to normal at the fda. Web 3 big fda approvals to watch for in q1 2022. Web the cder fast track (ft) approvals reports contain a list of approvals for fast track designated drugs. Phase3.bio places the fda review team’s deliberative process for drug evaluation into perspective so. Web fda calendar start free trial our fda calendar is designed to provide you with future catalysts across biotech & pharma. In the second quarter of 2023, the agency.Fda Approval Calendar Qualads
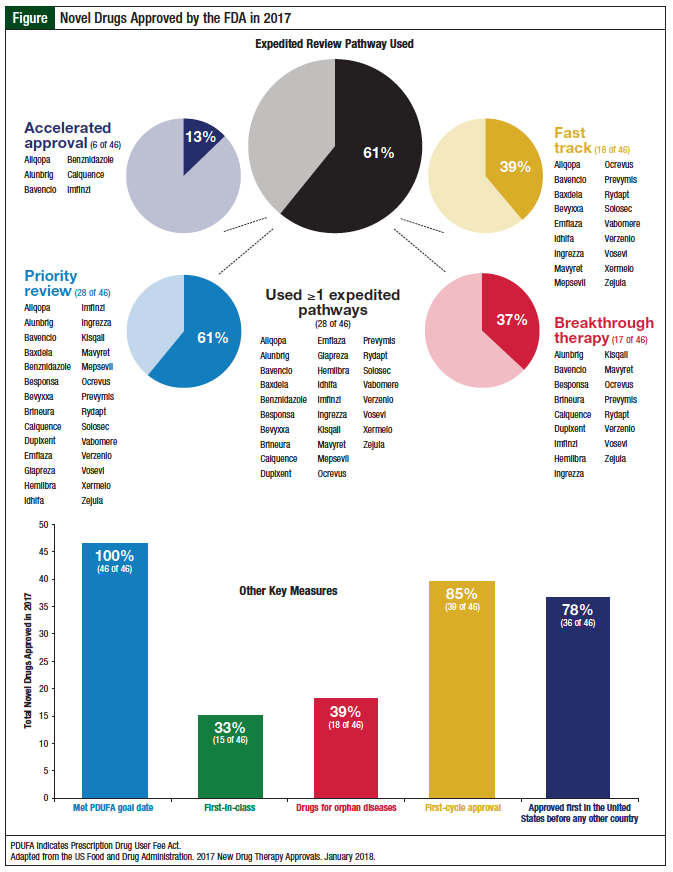
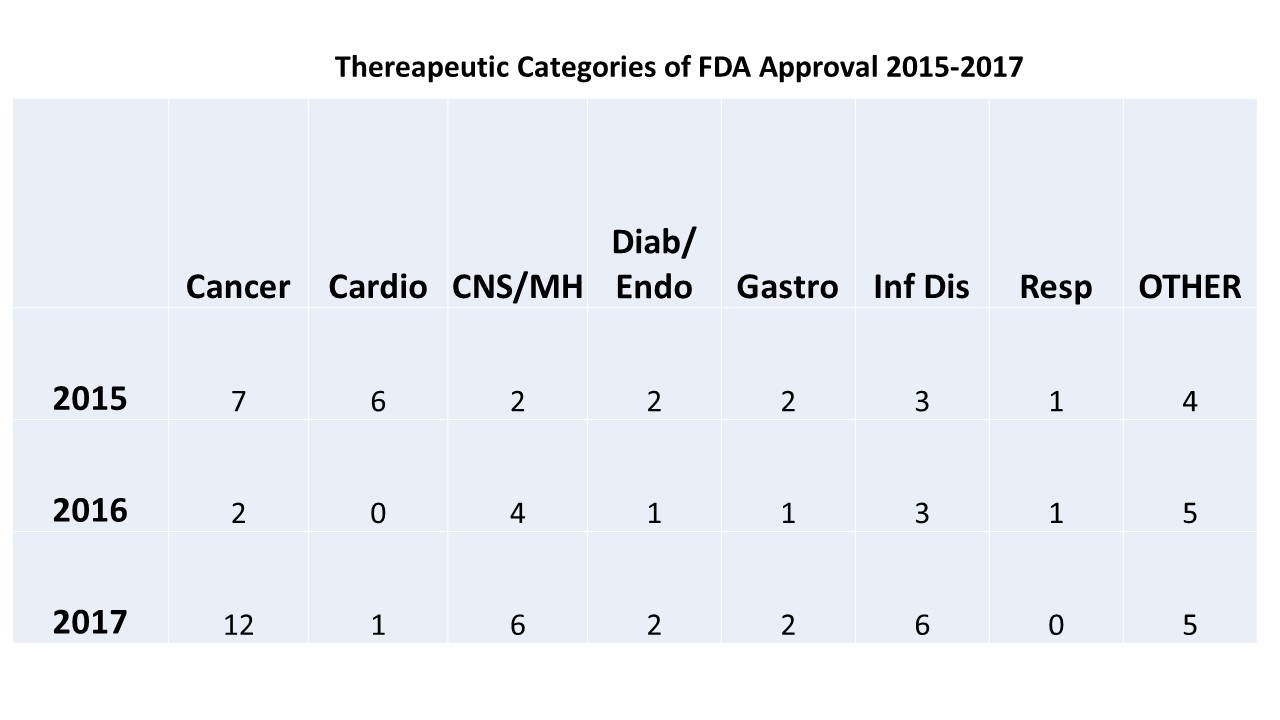
FDA Approvals in 2017 Represent a 21Year High Oncology Practice
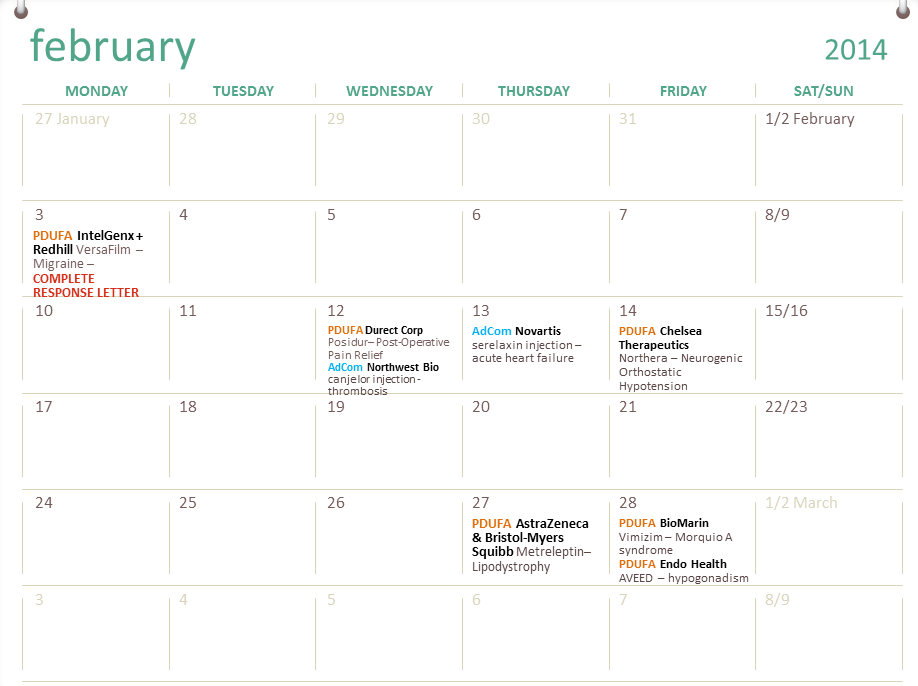
The Most Important New Drug Of 2014
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
Is FDA Set for Another Banner Year of Approvals? Eye on FDA
FDA Calendar FDA Tracker
Fda Generic Approvals 2015 takvim kalender HD
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
FDA Calendar FDA Tracker
FDA Calendar FDA Tracker
Related Post: