Fda Calendar Approval
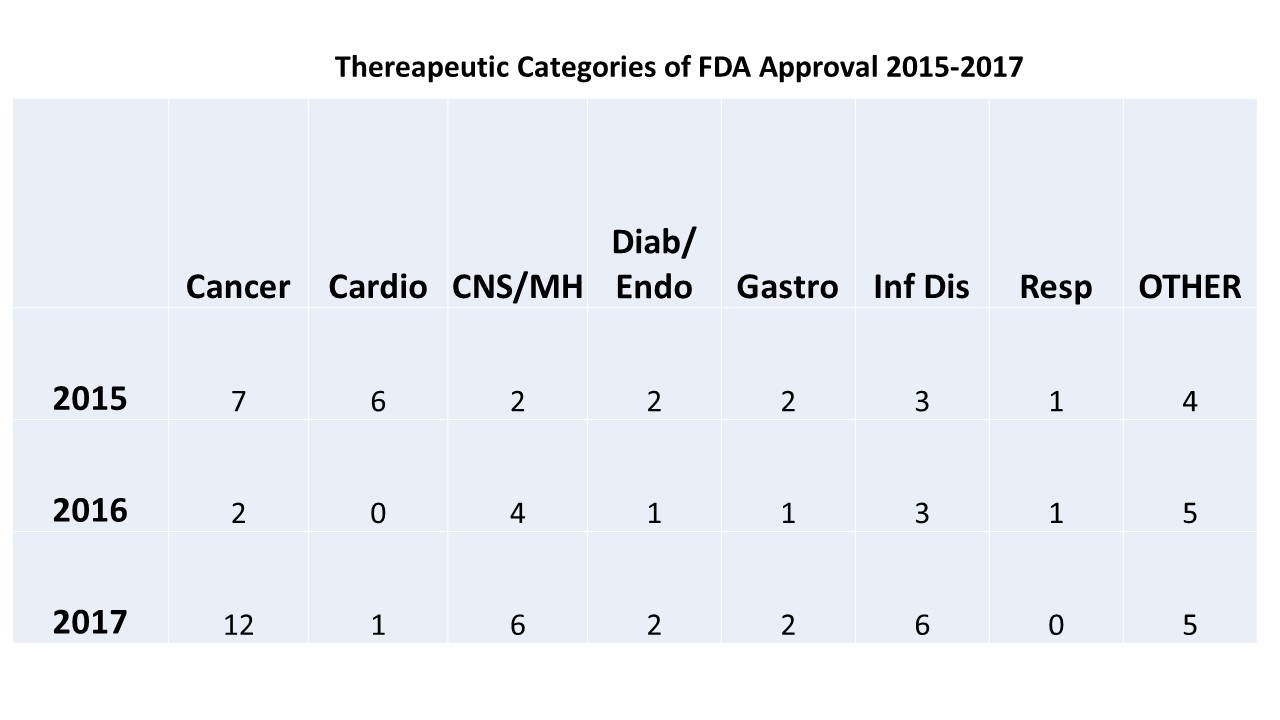
Fda Calendar Approval - Fda increased 20.48% compared to the first seven months of 2022, even with july. This calendar tracks upcoming pdufa drug approval dates and fda advisory committee meetings. Cder’s new molecular entities and new. Web 2023 device approvals. New drug application (nda) 07/23/2023. Web fda’s labeling resources for human prescription drugs; These calendars track upcoming pdufa drug. Web us fda approval and panel tracker: Web fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data. It lists significant meetings held by designated. Updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the. This public calendar is issued by the food and drug administration. It lists significant meetings held by designated. Web comprehensive suite of tools for trading and investing in biotech stocks. (regn) on friday announced that the. Web the fda pdufa calendar is a chronological calendar of pdufa target dates as well as advisory meetings (adcomm). Cder’s new molecular entities and new. Web cder drug and biologic approvals for calendar year 2022; This public calendar is issued by the food and drug administration. Web 2023 device approvals. Web cder drug and biologic approvals for calendar year 2022; Web this public calendar is issued by the food and drug administration. This public calendar is issued by the food and drug administration. Web fda’s labeling resources for human prescription drugs; It lists significant meetings held by designated. Updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the. It lists significant meetings held by designated. Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for. (regn) on friday announced that the. Web fda’s labeling resources for human prescription drugs; Fda calendar, pdufa date calendar, biotech company. This public calendar is issued by the food and drug administration. Last month the fda granted approval for the first and second. This calendar tracks upcoming pdufa drug approval dates and fda advisory committee meetings. New drug application (nda) 07/23/2023. Web this public calendar is issued by the food and drug administration. These calendars track upcoming pdufa drug. The products listed in this section include some of the newest medical technology from the. Web comprehensive suite of tools for trading and investing in biotech stocks. Web the fda pdufa calendar is a chronological calendar of pdufa target dates as well. Web fda’s labeling resources for human prescription drugs; Web a regulatory application (nda, snda, bla, sbla, etc.) is usually accepted for standard review or priority review. Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for. Web discover trading opportunities at every stage of the drug development pipeline. New drug application (nda) 07/23/2023. Web us fda approval and panel tracker: This public calendar is issued by the food and drug administration. Web this public calendar is issued by the food and drug administration. (regn) on friday announced that the. It lists significant meetings held by designated. It lists significant meetings held by designated. This calendar tracks upcoming pdufa drug approval dates and fda advisory committee meetings. Web comprehensive suite of tools for trading and investing in biotech stocks. Web 2023 device approvals. Web this public calendar is issued by the food and drug administration. Web discover trading opportunities at every stage of the drug development pipeline. Web this public calendar is issued by the food and drug administration. Cder drug and biologic approvals for calendar year. It lists significant meetings held by designated. Web comprehensive suite of tools for trading and investing in biotech stocks. It lists significant meetings held by designated. Web us fda approval and panel tracker: Web this public calendar is issued by the food and drug administration. Web this public calendar is issued by the food and drug administration. This public calendar is issued by the food and drug administration. Cder drug and biologic approvals for calendar year. Fda increased 20.48% compared to the first seven months of 2022, even with july. These calendars track upcoming pdufa drug. New drug application (nda) 07/23/2023. Web the fda pdufa calendar is a chronological calendar of pdufa target dates as well as advisory meetings (adcomm). It lists significant meetings held by designated. Web this public calendar is issued by the food and drug administration. Updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the. Cder’s new molecular entities and new. (regn) on friday announced that the. Web comprehensive suite of tools for trading and investing in biotech stocks. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or pdufa date. Web 2023 device approvals. Web the pdufa/fda approval calendar and finding potential fda approval catalyst dates biopharmcatalyst provides a pharmaceutical data bank. Web drug approvals by the u.s.FDA Calendar FDA Tracker
Fda Approval Calendar Qualads
FDA Calendar FDA Tracker
FDA Calendar FDA Tracker
Is FDA Set for Another Banner Year of Approvals? Eye on FDA
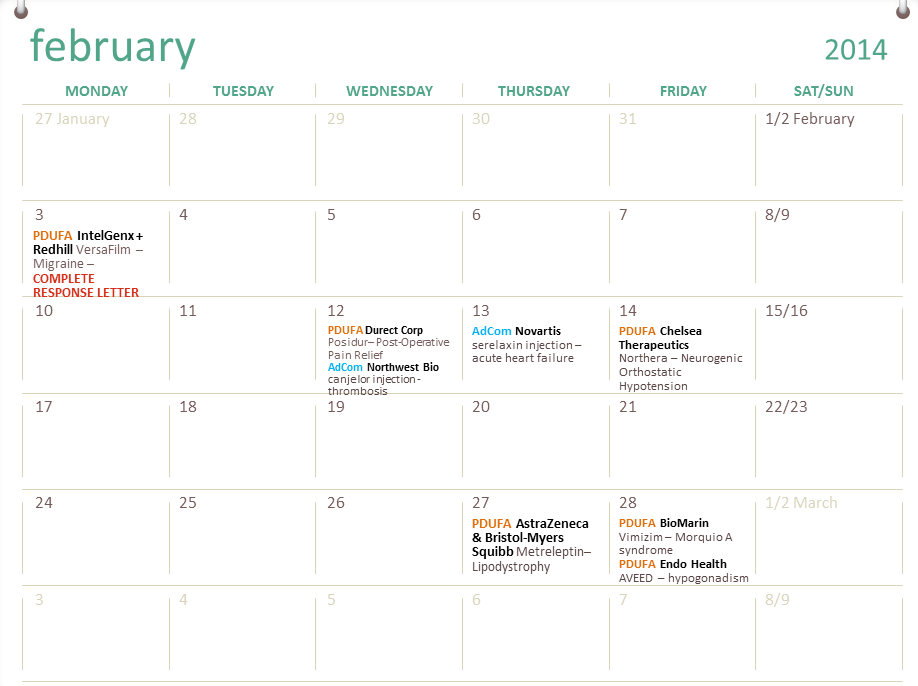
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
Pin on calendar Printable Online 2019
FDA Calendar FDA Tracker
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
The Most Important New Drug Of 2014
Related Post: